Your cells are constantly renewing and replicating - some faster than others. For example, intestinal cells turn over every three or four days, while the average lifespan of your skin cells is about a month. Lots of replication going on, constantly.
Every time a cell replicates, there's a chance that an error will occur in the nuclear DNA. Most errors are caught and fixed, but some errors slip through. Spontaneous mutations occur. DNA mismatch repair enzymes usually catch and fix the error, but if a spontaneous mutation occurs in a cell that is replicating, the mutation is carried on in that tissue.

Mutagens and carcinogens, such as X-rays and arsenic, can cause changes or breaks in the DNA strand as well. Oxidizing agents and alkylating agents can also cause DNA damage.
Now, a new study shows that epigenetic markers - methylation markers - increase the mutation rate, but then the mutation causes more methylation of surrounding regions.
First, a little more context…
Somatic mutations add up in aging:
The mutations that occur during cell replication are called somatic mutations. They occur in cells other than the sperm or egg, and they’re not inherited. We all end up with a mosaic of different mutations in cells throughout the body by the time we are old.
This progressive damage and accumulation of mutations is thought to be a fundamental part of aging.
A new study links together these somatic DNA mutations with another fundamental hallmark of aging - DNA methylation.
It’s a pretty cool study and sheds light on what causes aging at a fundamental level. You can read the full study as a preprint version here.
So what’s DNA methylation?
DNA methylation is a way that genes get turned off, or marked with a methyl group, so that they aren’t translated into their protein.
Genes —> transcribed to mRNA —> translated to proteins
Methyl marks cause a gene to not be transcribed. This is just one of many ways that genes are "turned on" or "turned off" for transcription.
Epigenetics is the general term for the different modifications that turn a gene on or off. DNA methylation is one way, and it is measured to determine biological age in epigenetic aging tests.
Epigenetic age tests for biological age:
There are a bunch of companies that offer different epigenetic age tests. Essentially, these give a snapshot of biological age based on epigenetic markers. (I’ve always been a little skeptical of the accuracy of these tests… This 2024 study explains that there is up to a 5-year ‘epigenetic age’ difference between testing in the morning compared to at night, and this study shows epigenetic age differs based on tissue type.)
Back to the new study…
DNA Methylation Increases Somatic Mutations
Researchers at UC San Diego used data from more than 9,000 individuals. They found that methylation of certain DNA regions called CpG sites increased the odds of a somatic mutation in that site by 16-fold. This was something that was previously known - the methylation of cytosine (the “C” in CpG) is prone to causing replication errors there. Here’s the full study link again, if you want to go read it.
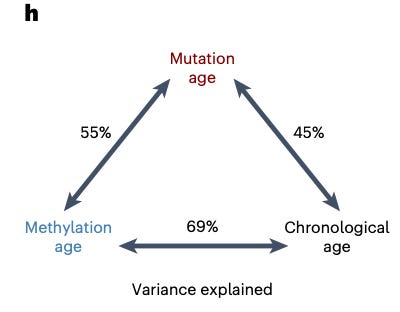
What was interesting was that the individual somatic mutations in the CpG site then tended to cause a large methylation change to the surrounding DNA region. About 15% of these somatic mutations ended up having a ripple effect of methylation of neighboring genes. Thus, these somatic mutations are at least partly responsible for the epigenetic changes seen during aging. The results showed that “somatic mutations explain almost 50% of the variation in methylation age across individuals”.
Why is this interesting?
First, it gives us a better understanding of what is going on at the cellular level in aging, but there are still a lot of questions here as to causality and direction.
It also suggests that hypermethylation of a DNA region causes an increase in mutations — and then further methylation. Turning off a gene that protects the cell against uncontrolled growth (e.g. cancer) can lead to tumors. Turning off genes that modulate the inflammatory response can lead to excessive inflammatory cytokines.
The bigger picture for longevity:
Much of the longevity research is currently focused on reversing epigenetic changes — essentially doing an epigenetic reset of cells to reverse aging. But if these epigenetic changes are a symptom (or result) of the mutation rather than an initial cause of aging, then a large focus of aging research may be, at least partly, going down the wrong path. There is probably still a theoretical way to reverse aging by restoring the entire cell's DNA to its original state.
Preventing mutations from occurring has also been a focus of healthspan as a way to prevent cancer.
A question that I’m left with:
Is an excess of methyl groups - e.g. from folic acid or methylfolate - going to increase DNA methylation and (slightly) accelerate aging? If you know the answer, please comment below or email me.
Personal takeaways:
I’ve always been of the mindset “what doesn’t kill you makes you stronger”. Perhaps avoiding exposure to even low levels of carcinogens and mutagens is more important than I realized.
Here are some of the ‘big’ mutagens:
Aflatoxin (a mycotoxin produced by certain types of mold, found on peanuts)
Radon (naturally occurring radioactive gas, causes lung cancer)
Ionizing radiation (X-rays, gamma rays, alpha particles)
Polycyclic aromatic hydrocarbons (e.g. cigarette smoke, smog)
Nitrosamines (tobacco, smoked meats, fish, bacon, beef jerky)
Nitrous acid (deaminating aged that causes transition mutations)
Bromine (in fire retardants, bromated flour and baked goods)
Benzo[a]pyrene (also in tobacco smoke and grilled meat)
Base analogues - these are things that mimic normal DNA bases (the A, C, G, and Ts), 5-bromouracil is an example
More generally, mutation in DNA can occur due to:
High levels of ROS due to cellular stress can cause mutations. This is something we are likely all dealing with as we age.
Metals such as arsenic, cadmium, chromium, and nickel — when they overwhelm your individual ability to detoxify.
Oncoviruses, such as HPV and hep B, and H. pylori bacteria
What can I do personally? Top on my list is avoiding arsenic (e.g. from rice grown in the US), reducing radon exposure, and cutting back on smoked meats and nitrosamine-containing meats stick out as big ones for me.
What about you? Which of these mutagens is adding to your epigenetic age?