A new pathway to target for Alzheimer's disease prevention
Genetics research on familial Alzheimer's highlights the importance of Reelin
Researchers have been working on understanding Alzheimer’s disease for my entire lifetime - and then some.
The different theories of Alzheimer’s kind of jumble together for me… I remember my grandmother worrying about aluminum foil because aluminum caused Alzheimer’s. Then came the decades of focus on amyloid beta, with the idea that clearing it out could prevent or reverse Alzheimer’s. In the back of my mind is that long-term Benadryl use could cause Alzheimer’s because it’s an anticholinergic. And then there are the dreaded Tau tangles, like a tangled wad of thread stuck in the brain.
None of that is quite right.
Let me lay out the theories on the causes of Alzheimer’s more logically:
Cholinergic hypothesis: Acetylcholine is a neurotransmitter. The idea here is that the decline in acetylcholine concentration and cholinergic activity leads to damage to cholinergic neurons, which are found in the region of the brain that is affected by Alzheimer’s.[ref] Cholinesterase inhibitor drugs are still used in Alzheimer’s.
Metal accumulation (aluminum, iron, zinc): Aluminum was the first metal identified (1911) as causing neurodegenerative problems, but the overall idea is that metals - zinc, iron, aluminum - accumulate in the brain and lead to oxidative stress and amyloid beta formation, resulting in neuronal death[ref][ref]
Amyloid hypothesis: Amyloid-beta peptides are produced from the cleavage of a larger protein called amyloid precursor protein (APP), which is then cleaved to produce smaller proteins. The formation of Aβ40 and Aβ42 causes amyloid fibrils to form plaques, which disrupt neuronal function leading to the death of the neurons. One of the early, major papers on this is from 1992.[ref]
Tau misfolding (tangles) hypothesis: Tau is a protein that is part of the structure, or cytoskeleton, of the neurons. In Alzheimer’s, tau becomes phosphorylated and forms aggregates, or tangles of insoluble protein, that disrupt neuronal function and lead to the death of neurons.
Tau as a prion: One theory is that tau tangles can spread from one neuron to another, spreading like a prion.[ref]
Neuroinflammation hypothesis: The activation of microglia (the brain’s immune cells) causes pro-inflammatory cytokine release and damage to the neurons. Microglia can be activated by the presence of amyloid-beta plaques, tau tangles, metal ions, persistent viruses, etc.
Which is correct? Are all of these hypotheses partially right but yet missing an initiating factor?
Let’s take a look at a compelling new player in the field of Alzheimer’s research that ties a lot of these theories together.
Using genetic research to understand the cause:
There are broadly two types of Alzheimer’s:
familial, early-onset Alzheimer’s caused by rare genetic mutations
late-onset Alzheimer’s, where common genetic variants add to the susceptibility but do not cause it entirely
Early-onset Alzheimer’s is caused by mutations in the APP, PSEN1, and PSEN2 genes. APP encodes amyloid peptides. PSEN1 and PSEN2 are involved in cleaving APP to produce amyloid-beta (Aβ) peptides. Thus, all of these point to an amyloid root cause for Alzheimer’s disease in people with early-onset Alzheimer’s.
Late-onset Alzheimer’s has a strong genetic link to a specific APOE type. Apolipoprotein E (APOE) is involved in transporting cholesterol and triglycerides in the brain.
Have genetic raw data from 23andMe? How to determine your APOE type (Be sure that you want to know, before checking!)
There are three common APOE alleles: E2, E3, and E4.
The E2 allele is protective against Alzheimer’s — carriers of the E2 allele are less likely to get Alzheimer’s disease, but some people with E2 alleles do get Alzheimer’s.
E3 is the ‘normal’ type, and people with the E3 allele are at a normal risk of Alzheimer’s.
The E4 allele is found in about 20-25% of the population and increases the risk of Alzheimer’s. However, not everyone with an E4 allele (or even with two copies of the E4 allele) will end up getting Alzheimer’s disease.
Rare mutations have been shown to protect against Alzheimer’s in people with familial Alzheimer’s disease, and these rare mutations can point us toward possible pathways to target for prevention. A couple of mutations in APP are associated with resistance to Alzheimer’s, an uncommon variant in the fibronectin gene decreases the risk in APOE4 carriers, a mutation in APOE protects against Alzheimer’s, and a rare mutation in the Reelin (RELN) gene prevents early-onset Alzheimer’s in PSEN1 mutation carriers.
The RELN - reelin - aspect of Alzheimer’s protection is what I’m going to focus on here.
Reelin’ in the Alzheimer’s pathology:
Reelin is a secreted glycoprotein with multiple functions in the body, including control of prenatal neuronal development and adult brain function. In adults, reelin affects brain function, memory, cognition, and the creation of new brain cells. It makes the adult brain dynamic and resilient.[ref]
Reelin synthesis and secretion:
The RELN gene encodes the reelin protein. The protein is first synthesized, then glycosylated, meaning sugar molecules are attached to it. The reelin protein is then cleaved into smaller proteins that activate specific receptors. Reelin is secreted in the brain by GABAergic neurons.
Receptors for reelin: APOER2 and VLDLR
Like many proteins, reelin can bind to a receptor to cause an action to take place.
There are two main receptors that reelin binds to: apolipoprotein E receptor 2 (ApoER2) and very low-density lipoprotein receptor (VLDLR).[ref] Binding to these receptors results in the activation of a protein in cells called DAB1, which is important for neural development and brain health.
ApoER2:
The ApoE receptor 2 is found in the central nervous system, brain, olfactory bulb, placenta, and testes.
Relevant here - in addition to binding with reelin, ApoER2 can bind to apolipoprotein E (ApoE), which is a cholesterol transporter in the brain. ApoER2 can also bind to selenoprotein P, resulting in the transport of selenium into the cell. [ref]
VLDLR:
The very low density lipoprotein receptor is similar to ApoER2. In addition to binding to reelin, VLDLR also binds to ApoE. However, the receptor is less utilized than the ApoER2 receptor. [ref]
NMDA:
Reelin interacts with the NMDA receptor via the ApoE receptor. The NMDA receptor is involved in glutamate (excitatory) neurotransmission. Studies show that Reelin enhances glutamate signaling through the NMDA receptor. [ref][ref][ref]
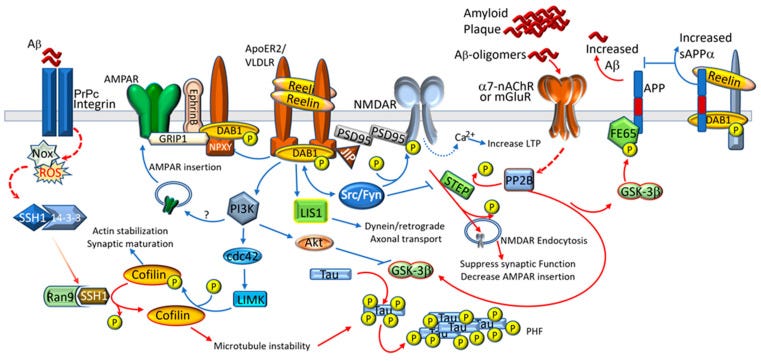
So… we have reelin as a glycoprotein that activates the same receptor as APOE and interacts with glutamate transmission — both of which are implicated in Alzheimer's disease. The glycoprotein is important for synaptic plasticity and healthy neurons in the adult brain.
A reelin mutation prevents early-onset AD:
In 2023, researchers found that a mutation in the RELN gene prevented early-onset Alzheimer's in someone with a PSEN1 mutation. This person should've gotten early-onset Alzheimer's around age 50, but remained cognitively intact until age 70. After he died, an autopsy showed that his brain had amyloid plaque but not tau tangles, associated with Alzheimer's disease. Researchers found a rare RELN gene mutation and made a mouse model with reduced tau tangles. [ref]
The RELN mutation that protects against Alzheimer’s is a gain-of-function mutation that increases reelin in the brain. This is a very strong indicator that higher Reelin levels may help to protect against Alzheimer’s.
Increased reelin and preventing Alzheimer’s:
While early-onset, familial Alzheimer’s is rare, research also shows that increased Reelin or increased Reelin-Dab1 activation is associated with a lower risk of Alzheimer’s disease. A deficiency of reelin increases the risk of Alzheimer’s[ref]
There are several ways that reelin interacts with AD:
Interaction with APOE E4:
Research shows that the APOE E4 allele impairs synaptic plasticity by reducing the recycling of the ApoER2 receptor to the cell surface. Thus - there aren’t as many ApoER2 receptors on the neurons in APOE4 brains. The E4 allele also reduces the NMDA (glutamate) receptor movement to the cell membrane. Without sufficient ApoER2 receptors, the positive effects of Reelin on the neurons are missing. Combined with the reduced effect on the glutamate receptors, the decreased Reelin receptor binding is part of what drives neuronal degeneration in Alzheimer’s.[ref]
Again, reelin binds to ApoER2, which activates Dab1, initiating a bunch of cellular actions that promote plasticity and growth in neurons.
A study in people with two copies of the APOE E4 allele (high risk of Alzheimer’s) showed that a DAB1 genetic variant reduced the risk of getting Alzheimer’s.[ref] This indicates that increasing DAB1 may also be a way of mitigating Alzheimer’s risk.
Excess glutamate in the synapse hurts the neurons:
Amyloid-beta plaque interferes with the availability of the NMDA glutamate receptors, which are thought to play a role in neuronal loss. Reelin can counter this through the interaction with ApoeR2 and the NMDA receptor. Researchers think that “at high concentrations of Aβ peptides, Reelin can no longer overcome the Aβ-induced functional suppression” of the NMDA receptor.[ref]
Neurofibril tau tangles:
Disruption of the ApoER2-DAB1 pathway has recently been discovered by NIH researchers to be at the core of the Tau neurofibrillary tangles that accumulate in the brain in Alzheimer’s patients. Part of the reelin-Dab1 signaling pathway, through activation of GSK3β, prevents Tau phosphorylation, essentially preventing the beginning of the Tau tangle. Disruption then allows for the Tau tangle to form. Disruption of the ApoER2-DAB1 pathway also affects synapse strength and the delivery of cholesterol to the neuron.[ref]
Amyloid beta:
The Amyloid beta peptide is generated from processing the APP protein. (APP mutations can cause early-onset Alzheimer’s.) Reelin interacts with amyloid beta peptides, and some studies show that it can inhibit amyloid beta plaque formation. Initially, amyloid beta can reduce the binding of reelin with the ApoE receptor 2, and this upregulates reelin expression in a feedback loop. This explains why, in the initial phases of Alzheimer’s disease, there is an increase in reelin production but not in receptor binding or signaling. It is thought that this then increases neurofibril tau tangles, causes glutamate excess in the synapse, and starts the cascade of events causing neuronal death.[ref]
Recap: Reelin interacts with many of the known pathways involved in Alzheimer’s disease - from amyloid beta to tau tangles to neuronal death. Moreover, there is a clear interaction with APOE E4.
Environmental toxins, dietary interactions:
Studies show that there are environmental factors that increase the relative risk of Alzheimer’s disease. Thus, anything that plays a causal role in Alzheimer’s disease should be affected by environmental factors.
Pesticide residue:
Chlorpyrifos is a commonly used organophosphate pesticide. In adult animals, chlorpyrifos exposure, along with low reelin, causes autism-like behavior.[ref]
In adult animals, glyphosate-based herbicides are associated with an upregulation of Reelin and Dab1, which indicates a compensatory mechanism in response to the herbicide.[ref]
Eating organic foods whenever possible may reduce exposure to pesticide residue.
Sufficient selenium:
The ApoER2 receptor not only binds to reelin, but it also moves selenium into the neurons. Selenium deficiency in the brain in animals causes irreversible neurological dysfunction and progressive neurodegeneration. Low reelin levels in the brain can downregulate ApoER2 receptor levels.[ref] Multiple studies show that people with Alzheimer’s have low selenium levels in their brains.[ref]
Good sources of dietary selenium are Brazil nuts and seafood. The US RDA is 55 mcg/day for most adults, and the upper daily limit is 400 mcg/day.[ref] Selenium is needed in the right amount — too much can also be toxic.
High-fat diet (in animals):
In mice fed a high-fat diet, the expression of the Reelin receptors (both ApoER2 and VLDLR) decreased in the hypothalamus region of the brain.[ref]
The research on high-fat diets and Alzheimer’s is contradictory[ref][ref][ref], so take this interaction as just a possibility that needs to be further researched.
Possible solutions?
Let me be upfront here - none of these solutions are going to cure Alzheimer’s disease.
In mice, researchers can inject reelin into the brain and see the positive effects.[ref] It isn’t clear whether that would work in humans, and excess peripheral reelin may have negative effects, such as increasing clotting and inflammation in the endothelium. [ref]
Supplements that upregulate reelin include:
Bacopa monniera:
Bacopa monniera is a herbal supplement known for improving learning and memory. A study in animals showed that Bacopa monniera supplement upregulated reelin by reducing its DNA methylation. Bacopa supplementation also caused increased ApoER2 and activation of the NMDA receptor.[ref] Bacopa supplements are also neuroprotective in animal models of Alzheimer's.[ref][ref] A small clinical trial in Alzheimer’s patients showed that an herbal supplement that contained Bacopa improved cognitive function in AD.[ref]
Pinocembrin:
Pinocembrin is a flavonoid found in honey and propolis. Studies in animals show that pinocembrin improves memory impairment in a model of dementia by inducing the expression of reelin, dab1, and ApoER2.[ref]
What about Dab1?
The activation of Dab1 inhibits GSK3β, which then prevents tau tangles. Lithium orotate also inhibits GSK3β, and low-dose lithium has many studies on it for Alzheimer’s. Here’s my article on the studies on lithium microdosing for Alzheimer’s prevention.
Conclusion:
I still have more questions here, but I think that what is known so far points to an integral role of this pathway in brain health.
Reelin levels decrease in aging, which ties in with the biggest risk factor for Alzheimer’s, which is age.[ref]
The fact that a mutation that increases reelin is protective against early-onset Alzheimer’s is an important piece of the puzzle.
The interaction between reelin, ApoE, glutamate, amyloid beta, tau tangles — all point to the essential role of the reelin-Dab1 pathway.
The environmental factors and supplement interactions all fit with the idea that increasing reelin could help with Alzheimer’s prevention.
There seems to be quite a bit of interest in reelin and Dab1 right now, and hopefully, there will be more answers and real solutions soon.



There is a great deal of information in your write up, wow! You mention 23 and me, the company just filed for bankruptcy. I would like to add some information to "I remember my grandmother worrying about aluminum foil because aluminum caused Alzheimer’s." My mother in law had late onset Alzheimer's and when my husband learned there was no treatment being a scientist he started reading the research. That was 12 years ago. What he learned shocked him but also brought him hope and he was able to help his mother. She lived to age 97 and was not in end stage Alzheimer's. Research has reached a tipping point and aluminum is the cause of Alzheimer's. The good news is you can remove the aluminum by drinking silica rich mineral water. There are many waters that have enough silica to remove aluminum, Volvic, Fiji, Acilis are a few examples. My husband Dennis N Crouse using Causal Inference proves that aluminum is the cause of all the biomarkers which have been identified for diagnosing Alzheimer's. I am passionate about sharing this information with people. My husband and I have a website, you tube channels, blogs on blogger. I am active on substack and facebook. I can posts links if that is OK. I should also add that in areas of the world which have been designated as being Blue Zones (areas where people live longer compared to surrounding areas) the drinking water is rich in Silica. My husband has written an evidence based book on this subject.